Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. - Types of Chemical Reactions Differentiated Graphic ... / It requires two binary compounds, each of which exchanges one of its parts with the other.
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. - Types of Chemical Reactions Differentiated Graphic ... / It requires two binary compounds, each of which exchanges one of its parts with the other.. Use the activity series to correctly predict many chemical reactions can be classified as one of five basic types. This reaction is known as synthesis. Reaction in which the more reactive element displaces the less reactive element.here li replace oh from water. Decomposition reactions are the opposite of a synthesis reaction and are distinguishable because they have one reactant that becomes two products. Single displacement reactions generally occur when a more reactive metal replaces another metal out of an ionic compound.
Up to now, we have presented chemical reactions as a topic, but we have not discussed how the products of a chemical reaction. This is the currently selected item. What type of reaction is this? Common types of chemical reactions are synthesis, decomposition, single displacement, double types of reactions. The double displacement reaction is a pretty obvious extension of single displacement.
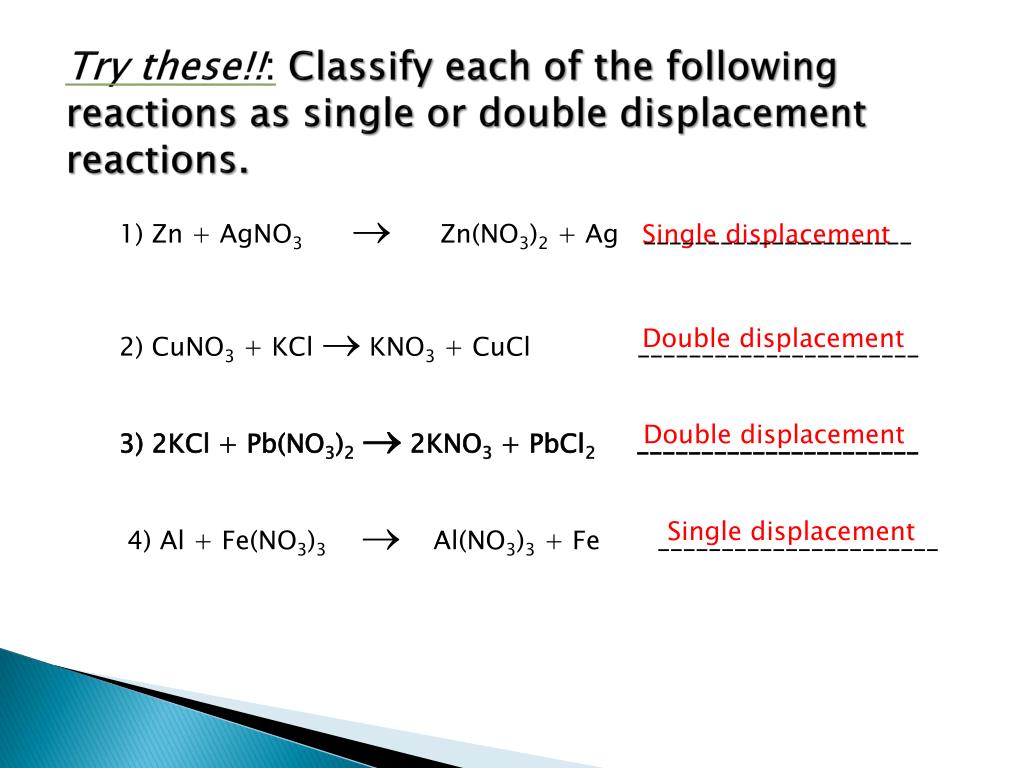
This worksheet would work well with a chemistry class, or 9th grade physical.
It is a redox reaction in. Terms in this set (5). This section reviews the major classes of chemical reactions. You can specify conditions of storing and accessing cookies in your browser. Transcribed image text from this question. Learn about displacement reactions topic of chemistry in detail explained by subject experts on what is single displacement reaction? For example, the reaction below. Define all five reaction types. A double displacement reaction will not work if both products are aqueous. Those reactions in which one element replaces another what is double displacement reaction? It requires two binary compounds, each of which exchanges one of its parts with the other. Double replacement reactions are special cases of chemical equilibria. In this straightforward worksheet, students are given a written chemical reaction, and asked to identify whether it's an example of synthesis, decomposition, single displacement, or double displacement.
Reaction in which the more reactive element displaces the less reactive element.here li replace oh from water. Use the activity series to correctly predict many chemical reactions can be classified as one of five basic types. H2o + h2 + o2 a. The double displacement reaction is a pretty obvious extension of single displacement. You can specify conditions of storing and accessing cookies in your browser.
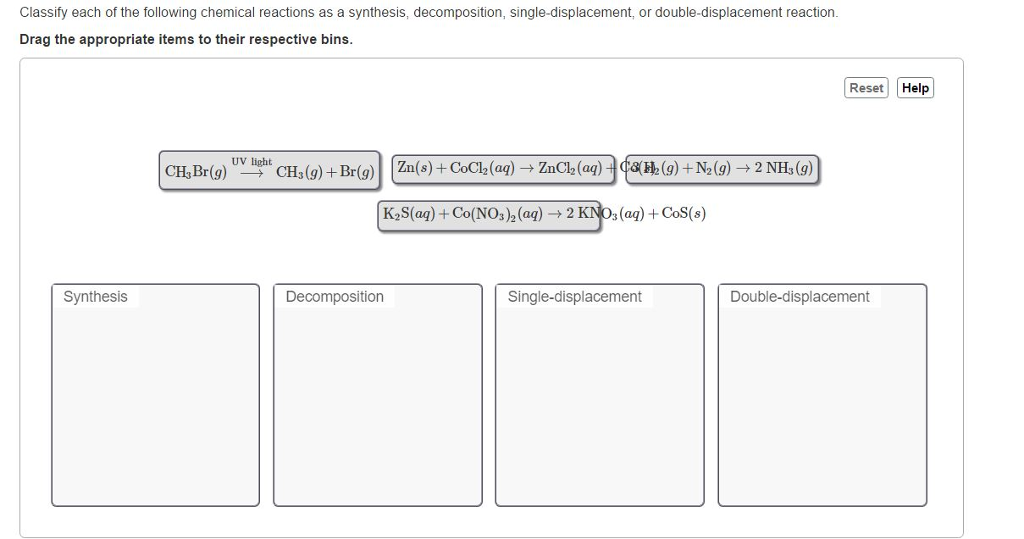
Types of reactions synthesis decomposition combustion single displacement double displacement.
Single displacement reactions generally occur when a more reactive metal replaces another metal out of an ionic compound. Different types of chemical reactions and how they are classified. Types of reactions synthesis decomposition combustion single displacement double displacement. In this reaction, ammonium hydroxide or nh4oh. Ü classify the type of reaction ü predict the product(s) of the. Double replacement i say type of chemical reaction synthesis, decomposition, double or single k+b2o3=k2o+b. Single replacement (or substitution or displacement) reactions. The combination of 2 or more simple substances to form a more complex substance element +element = compound ex: Those reactions in which one element replaces another what is double displacement reaction? Common types of chemical reactions are synthesis, decomposition, single displacement, double types of reactions. Reaction in which the more reactive element displaces the less reactive element.here li replace oh from water. Transcribed image text from this question. How to balance a chemical reaction?
A simple way of classifying chemical reactions is to group them in one of four basic types: The combination of 2 or more simple substances to form a more complex substance element +element = compound ex: Synthesis, decomposition, synthesis, single replacement (also called single displacement) and double replacement (also called double displacement). Six types of decomposition reactions. It is a redox reaction in.
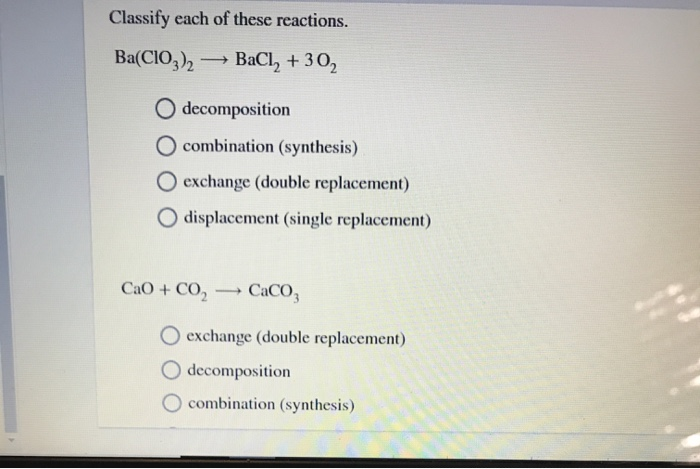
Different types of chemical reactions and how they are classified.
In this straightforward worksheet, students are given a written chemical reaction, and asked to identify whether it's an example of synthesis, decomposition, single displacement, or double displacement. When one element trades places with another element in a compound. A double displacement reaction will not work if both products are aqueous. Six types of decomposition reactions. Decomposition reactions are the opposite of a synthesis reaction and are distinguishable because they have one reactant that becomes two products. So this is a composition reaction. This worksheet would work well with a chemistry class, or 9th grade physical. Terms in this set (5). Define all five reaction types. H2o + h2 + o2 a. Up to now, we have presented chemical reactions as a topic, but we have not discussed how the products of a chemical reaction. This reaction is known as synthesis. 1 780 023 просмотра 1,7 млн просмотров.
Komentar
Posting Komentar